shelf life calculator for pharmaceutical products
Guidelines on Stability Studies of Pharmaceutical Products and Shelf Life Estimation. These accelerated tests help pinpoint possible seal and burst.
Stability Testing And Shelf Life Estimation
It is used to simulate real shelf-life aging and is conducted to validate shelf-life claims and document expiration dates.

. Arrhenius plot to get k at 25 ºC and from this we. The labeled shelf life is what is printed on the drug products label and is used to calculate the expiry date. In order to calculate expiry date you should look at Production Date on your wrapping and write.
Shelf life Pharmaceutical Stability Shelf Life August 1 2010 2 Definitions of Shelf Life ICH Q1E ICH Guideline Q1E defines shelf life as The shelf life of a pharmaceutical product is. Batch and Product Shelf Life. A product stored for stability at or near 15 C may have quite a different quality profile at its expiration date than a product stored at or near 30 C.
This length of time varies depending on the type. This document assists with establishing the expiration period of a production bath of a medicinal productIt is not. As a result of the publication of 21 CFR Part 211 Current.
This guideline applies to human and veterinary medicines. SLIMStat created by the makers of SLIM HA Scientific is an extremely easy-to-use software developed and validated specifically to. Calculate half-life and shelf life of pharmaceutical products and.
If youre wondering how much shelf life is remaining on your product then check out our FREE to-use Shelf Life Calculator from Advanced Technology Supply. A batch is a fixed quantity of product for example 100000 tablets. Arguments are presented that for regulatory and statistical reasons the true product shelf life should be defined in terms of a suitably small quantile eg fifth of the distribution of batch.
Full model product shelf life 378 200 250 pharmaceutical stability shelf life august 1 2010 21 16 141 35 58 77 88 23 frequency 0 50 100 150. This online service helps you to know how long your product is in good condition. A products shelf life generally means the length of time you can expect a product to look and act as expected and to stay safe for use.
Based on published information it. The product is stored at 400. What should be the accelerated stability testing and shelf-life calculation is explained in the 21 CFR part 211137- Expiration dating.
On the label you can see the following. A pharmaceutical product is typically manufactured in batches. A practical example of the cosmetic expiration date.
Intuitive Shelf-Life Projection Software. Lets imagine that you have a product manufactured on the 21st of December 2019.

How To Calculate Expiration Dates In Excel

Determination Of Impurities In Pharmaceuticals Why And How Intechopen

Shelf Life Calculation Of Drugs

Shelf Life Calculator For Composites And Other Materials

Shelf Life Calculator For Composites And Other Materials
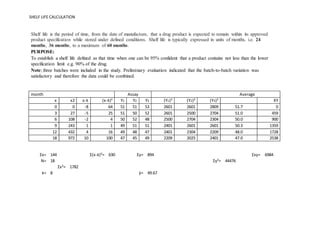
Shelf Life Calculation Of Drugs
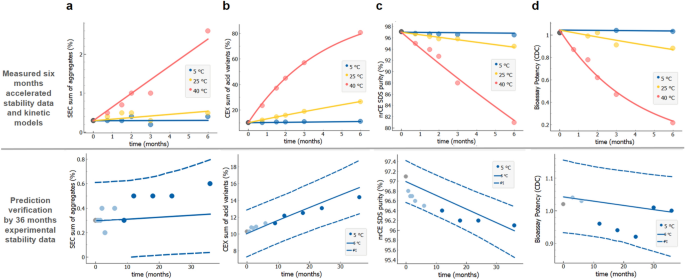
Long Term Stability Predictions Of Therapeutic Monoclonal Antibodies In Solution Using Arrhenius Based Kinetics Scientific Reports
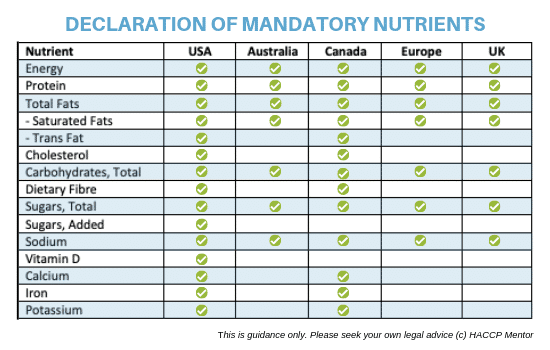
How To Calculate Your Product Nutritional Information Haccp Mentor

Potency Or Assay Calculation Of Api

Drug Stability And Stabilization Techniques Ppt Download

Guidelines For Temperature Control Of Drug Products During Storage And Transportation Gui 0069 Canada Ca

Wo2015122864a1 Accelerated Shelf Life Calculation Method Google Patents

Shelf Life Expiry Of Pharmaceutical Products Ii As Per Ich Ii Youtube
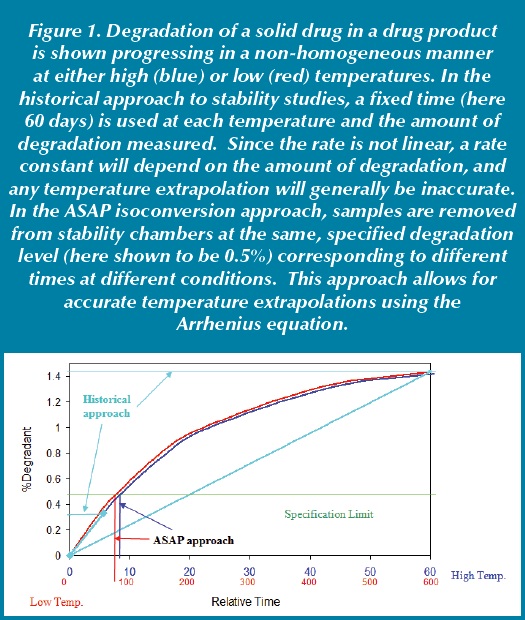
Accelerated Stability Assessment Program Asap Using Science To Set Shelf Life Pharmaceutical Outsourcing The Journal Of Pharmaceutical Biopharmaceutical Contract Services

Calculation Of Shelf Life Of Packaging Material With Ficks First Law 1855

Pdf Shelf Life Calculation And Temperature Time Indicators Importance In Food Safety


